Explain How the Acids Listed Below Are Alike and Different
Hydrochloric acid is a clear highly corrosive strong acid. Fatty acids are major components of fats and oils.
Each amino acid has 4 different groups attached to α- carbon.

. There are many different ways that DNA can be changed resulting in different types of mutation. Substitution A substitution is a mutation that exchanges one base for another ie a change in a single chemical letter such as. On the left the amino group.
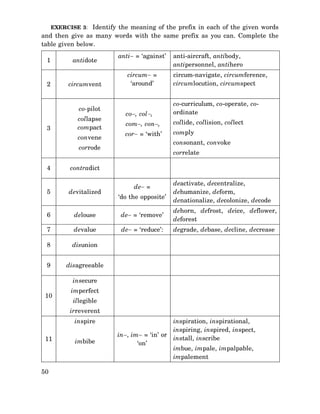
See back of book for one-letter amino acid code. 1 non-polar and neutral 2 polar and neutral 3 acidic and polar 4 basic and polar. Three fatty acids join with a.
Glutamine - Gln - Q. The main difference between amino acids and fatty acids is that the amino acids are the building blocks of proteins whereas the three fatty acids bound to a glycerol and form a triglyceride which is the main constituent of fat. - Explain how the acids listed below are alike and different.
Its found in diluted form as muriatic acid. The carboxylic acids have pK a s near 45 and the conjugate acid of the amine has a pK a of 10. Amino acid fatty acid folic acid.
FAMILY VW is a convenient mnemonic for hydrophobic amino acids A. Arginine - Arg - R Lysine - Lys - K Aspartic acid - Asp - D Glutamic acid - Glu - E Polar form hydrogen bonds as proton donors or acceptors. Essential amino acids also known as indispensable amino acids are amino acids that humans and other vertebrates cannot synthesize from metabolic intermediates.
There are basically four different classes of amino acids determined by different side chains. They differ from each other in their side-chain called R group. Fatty acids have a long chain hydrocarbon stuck on to a carboxylic acid.
A conjugate acid is formed when a proton is added to a base and a conjugate base is formed when a proton is removed from an acid. Answer 1 of 9. Hydrocarbon chain of the fatty acid can be saturated no double bonds between carbon atoms or unsaturated there are double bonds between carbon atoms.
- Amino acids are weak acids - All have at least 2 titratable protons. I T P I Y F G P M A G V I G T P. Below the 20 most common amino acids in proteins are listed with their three-letter and one-letter codes.
Charged side chains often form salt bridges. Consider the following reaction CH 3 COOH H 2 O CH 3 COO H 3 O. What are hydrophobic and polar groups.
The hydrocarbon chain can be of any length and of any degree of saturationfully saturated monounsaturated di or poly unsaturated. Which of the three 20-amino-acid sequences listed below is the most likely candidate for such a transmembrane segment. Weak acids like CH 3 COOH are partially dissociates and give fewer amounts of protons.
The second one is a carboxyl group -OOOH. Some proteins function as enzymes some as antibodies while others provide structural supportAlthough there are hundreds of amino acids found in nature. Here is a quick summary of a few of these.
Amino acids are organic molecules that when linked together with other amino acids form a proteinAmino acids are essential to life because the proteins they form are involved in virtually all cell functions. On the right the acid group. I T L I Y F G V M A G V I G T I LLIS B.
The only difference is that the solution does not have to be water. Shown below as fully protonated species and therefore have 2 pKas o α-carboxyl -COOH o α-amino -NH. 7-The essential amino acids are eight.
Key Difference Fatty Acid Synthesis vs Beta Oxidation. By contrast it is very high melting with decomposition insoluble in organic solvents and a million times weaker as an acid than ordinary carboxylic acids. The part of the amino acid that distinguishes it from other amino acids is called the R group.
To check whether a substance is an acid or not we can use several indicators like litmus paper or pH paper. Shown at the right is the structure. These 4 groups are.
These amino acids must be supplied from an exogenous diet because the human body lacks the metabolic pathways required to synthesize these amino acids12 In nutrition amino acids are. 5-Regulate brain activity such as alertness and feelings of depression 6-Produce and store energy. The acid and base which differ by proton are said to form conjugate acid and base pair.
Amino Acids as Acids Bases and Buffers. It gives an indication of the ability to lose a proton of a weak acid. A fatty acid is a carboxylic acid composed of a long hydrocarbon chain and a terminal carboxyl group.
The greater the electronegativity difference between atoms in. Muriatic acid for industrial purposes typically is 20 to 35 percent hydrochloric acid while muriatic acid for household purposes ranges between 10 and 12 percent hydrochloric acid. This atom is surrounded by three chemical groups.
There are 20 naturally occurring amino acids and all have common structural features an amino group -NH3 a carboxylate -COO- group and a hydrogen-bonded to the same carbon atom. We can still refer to the exact same acids as listed for the Arrhenius acid examples but this time well change the solvent to ammonia alcohol or anything else. In the pH scale from 1-6 acids are.
They are different chemically. Each amino acid has a different R group which can be something as simple as a hydrogen as seen in. Furthermore the R group of amino acids can contain atoms other than carbon and hydrogen while the R group of fatty acids only contains.
All amino acids contain a carbon atom in the middle of the molecule the alpha-carbon. The nine amino acids that have hydrophobic side chains are glycine Gly alanine Ala valine Val leucine Leu isoleucine Ile proline Pro phenylalanine Phe methionine Met and tryptophan Trp. Phenylalanine tryptophan lysine methionine threonine isoleucine leucine and valine.
The simple amino acid alanine is the last entry. This is the variable radical group and is different for every amino acid. Amino acids are grouped according to what their side chains are like.
The third group is denoted by R. A Bronsted-Lowry acid like an Arrhenius acid is a compound that breaks down to give an H in solution. One is an amine group -NH 2.
3 - Some amino acids have a thirdtitratable proton in the R group and therefore a third pKa o Showing all protonated. K a is the acid dissociation constant. The chemical has many industrial and lab uses.
Explain the reasons for your choice.

Help Late Homework Explain How The Acids Listed Below Are Alike And Different Amino Acid Brainly Com


No comments for "Explain How the Acids Listed Below Are Alike and Different"
Post a Comment